A week after Apple held an event revealing its new iPhone, Apple Watch and AirPod versions, the united state Fda has actually authorized an Apple Watch feature that can identify rest apnea in device-wearers.
The rest apnea discovery device comes 4 days prior to the launch day of Apple’s brand-new Collection 10 watch, which will certainly be launched on September 20. Those with existing Apple Watch Collection 9 and View Ultra 2 versions can make use of the rest apnea attribute beginning today, nevertheless, with download of Apple’s recently released watchOS 11 software program.
Individuals that experience rest apnea continuously quit and begin taking a breath over night and can, therefore, really feel worn out in the early morning. Left unnoticed, rest apnea can create heart issues and various other issues, in severe situations. Loud snoring can often be a signs and symptom. The problem impacts an approximated 1 billion individuals around the world and is commonly undiagnosed, according to wellness companies including the American Heart Organization.
According to the FDA’s approval notice, the Apple Watch rest apnea feature is not an analysis device, yet instead a non-prescription gadget developed to examine watch-wearers’ threat of rest apnea. Apple says the brand-new wellness attribute, Breathing Disturbances, is a statistics that functions as an “smart guardian for individuals’ wellness.”
The attribute functions making use of an accelerometer on one’s guard that discovers “tiny motions at the wrist related to disruptions to typical respiratory system patterns” throughout rest, according to Apple. Every 1 month, Apple Watch evaluates Breathing Disruption information and informs those individuals with a constant pattern of rest disruptions to go to a doctor for feasible medical diagnosis of rest apnea.
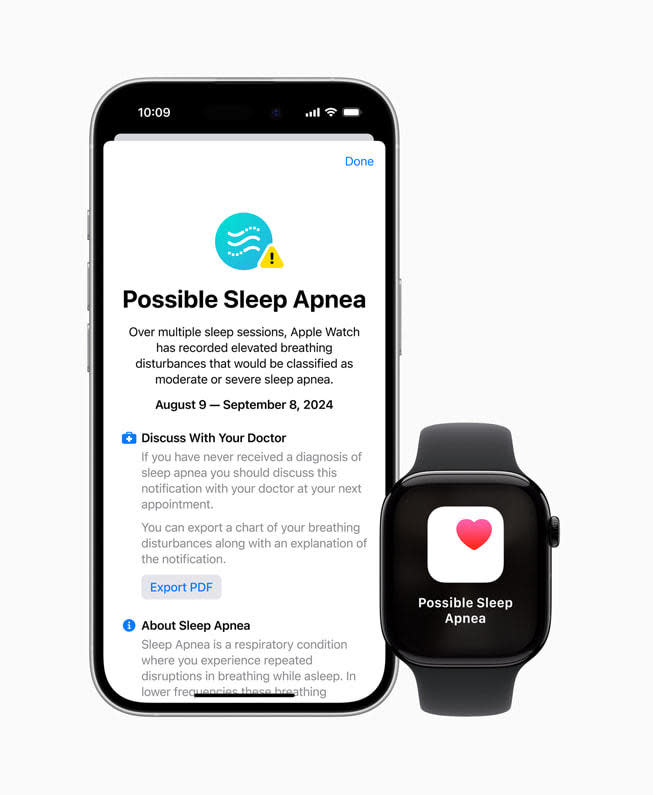

” Encouraging customers almost everywhere to have the capability to accurately recognize the visibility of uncommon breathing patterns throughout rest can assist discover a woefully underdiagnosed and severe clinical problem such as rest apnea,” Sairam Parthasarathy, teacher of medication at the College of Arizona and supervisor of Health and wellness Sciences Facility for Rest, Circadian and Neurosciences Facility for Rest, UA Health and wellness Sciences, stated in a declaration. “This is a significant advance in enhancing public wellness.”
The FDA’s green-lighting of the rest apnea attribute begins the heels of the company’s authorization of Apple’s AirPods Pro 2 as over-the-counter hearing aids for individuals with moderate to modest listening to loss, as the technology titan even more seals itself as a gamer in the wellness technology market. Hearing specialists applauded the company’s authorization of the brand-new earbuds as a favorable for the OTC hearing market and for the about 30 million Americans that experience moderate to modest listening to loss.
Tito Jackson, founding member of Jackson 5, dies at 70
Starter homes increasingly out of reach for American families
TikTok in court over U.S. ban: Breaking down the legal arguments
 Ferdja Ferdja.com delivers the latest news and relevant information across various domains including politics, economics, technology, culture, and more. Stay informed with our detailed articles and in-depth analyses.
Ferdja Ferdja.com delivers the latest news and relevant information across various domains including politics, economics, technology, culture, and more. Stay informed with our detailed articles and in-depth analyses.
